Suspended contaminants are found in the air that can affect our product or become toxic to personnel because in each industrial process liquids evaporate and dust and gases are generated that can affect health.
More than 40 years ago, the technology that helps us achieve adequate conditions of environmental cleanliness, known as clean rooms, was created.
We understand by clean rooms the place specifically created to maintain controlled levels of air pollution in spaces that are used mainly in the medical, pharmaceutical and electronic industries.
For the medical and pharmaceutical industry, these rooms help in the manufacture and packaging of medicines or supplies, operating rooms, biotechnology laboratories, for example.
And the electronics industry manufactures products such as semiconductors, displays, and storage devices.
The intention is that, within these rooms, the level of particles in the air can be controlled to sterilize the room.
For example, a clean room designed for the medical and pharmaceutical industry should be optimized to create a sterile manufacturing environment and provide a controlled environment.

Clean rooms need controlled environmental parameters in 6 aspects: amount of particles in the air, flow and volume of air, lighting and controlled temperature and humidity, as indicated by the standard ISO 14644-1.
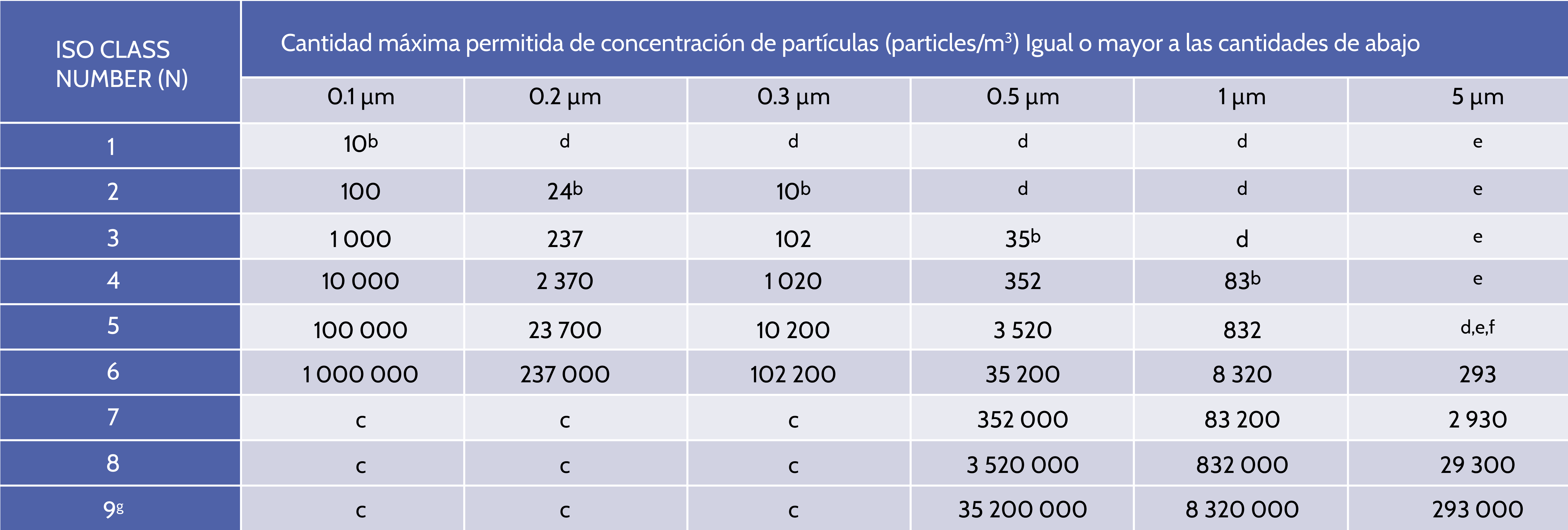
ISO 14644-1 is the most recognized standard around the world that applies to a variety of industries such as pharmaceuticals, food production, medical devices and aerospace manufacturing and classifies the cleanliness of the air for clean rooms and controlled environments according to the number or concentration of particles in the air volume.
Clean rooms are classified by the number of particles found in the air, according to ISO 14644. The number of particles equal to or greater than 0.5 mm is measured and that The result is used to classify the cleanroom. </span >
At Techmaster de México we offer you the Testing service for Controlled Environments with the capacity for Clean Room Testing. With it, you help your company comply with cleanroom certifications, including laminar flow hoods, biological safety cabinets, and extractor hoods.
Our accreditation scope under the standard ISO/IEC 17025:2017 and ANSI/NCSL Z540-1-1994 (R2002) integrates tests for controlled environments in compliance with ISO 14644:2015.












